-
 System CertificationZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and enhances customers' ability to respond to risks and...
System CertificationZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and enhances customers' ability to respond to risks and... -
 Product CertificationZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and enhances customers' ability to respond to risks and...
Product CertificationZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and enhances customers' ability to respond to risks and...
-
 Public DocumentsZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and enhances customers' ability to respond to risks and challenges, as well as their sustained commercial value, to win trust!
Public DocumentsZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and enhances customers' ability to respond to risks and challenges, as well as their sustained commercial value, to win trust! -
 Case PresentationZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and...
Case PresentationZOHOCERT establishes trust with excellent quality, conveys trust with the concept of Zhongzheng, and...


Certification Description
■ISO13485 medical device quality management system certification
ISO13485:2016 standard was officially released on March 1, 2016. The new ISO 13485:2016 standard introduces and strengthens a large number of best practices in the medical device industry. It is compatible with the requirements of national medical device regulations including the US FDA QSR 820, EU MDR & IVDR and China CFDA "Quality Management Regulations.
ISO13485, the medical device quality management system, is the world's medical equipment manufacturers most adhere to the standard. On the basis of ISO 9001, this standard increases the formulation of special requirements for the medical device industry, supports and helps some enterprises that manufacture or use medical products and services to reduce unpredictable risks, and plays a good role in promoting the quality of medical devices to achieve safety and effectiveness.
ISO13485: The main feature of the 2016 standard is that the ISO13485 is an independent standard, not a guide for the implementation of ISO9001 standards in the medical device industry, and the two are not compatible.
■Applicable industry
1. Medical product manufacturers and service providers that fulfill international, European and national legal and regulatory requirements
Enterprises that wish to implement a documented management system according to this standard.
3. Enterprises that develop, manufacture and sell medical equipment
Companies that want to demonstrate their ability to compete and perform in international, European and domestic markets.
■Application Information
1. Application for product quality certification and quality system certification signed by the authorized representative of the applicant;
2. The quality manual of the applicant unit, and the procedure documents of the enterprise if necessary;
3. The product for which certification is applied or the product standard covered by the quality system;
4. The standards declared by the applicant;
5. Medical device product registration certificate (copy);
6. Summary of the whole process of product production, description of product production process, special process and key process;
7. Product sales and user feedback in the past three years;
8. List of major purchased and outsourced parts;
9. Other materials, such as enterprise product catalogues, product profiles, product promotional materials, etc.; information on organizations and personnel who have provided certification consulting.
■ISO13485
1, improve and improve the management level of enterprises, avoid legal risks, increase the visibility of enterprises;
2, improve and ensure the quality of products, so that enterprises can obtain greater economic benefits;
3, is conducive to the elimination of trade barriers, access to the international market pass;
4, is conducive to enhance the competitiveness of products, improve the market share of products.
5, through effective risk management, effectively reduce the risk of product quality accidents or adverse events.
6. Improve employees' sense of responsibility, enthusiasm and dedication.
■ZOHOCERT
Is a professional service organization that has been deeply involved in the IT information industry for many years.
Has a number of senior academic management experts with more than 20 years of practical experience.
High-quality IT expert team services with a nationalized vision
Scope of Certification
Medical Device Quality Management SystemindustryServices
Standard for certification: ISO 13485:2016 "Medical device quality management system for regulatory requirements"
| Business scope (broad category) | Business Scope (Medium) | Professional Category Code |
|---|---|---|
| Passive Medical Devices | Design, development and production of passive medical devices (contact with human body) | 01.01 |
| Design, development and production of passive medical devices (non-contact human body devices) | 01.02 | |
| Active Medical Devices | Design, development and production of active medical devices (contact with human body) | 02.01 |
| Design, development and production of active medical devices (non-contact human devices) | 02.02 | |
| Sales | Wholesale and retail of medical devices | 03.01 |
| medical devices | Textiles and Textile Products | 04 |
| leather and leather products | 05 | |
| Wood and Wood Products | 06 | |
| Chemicals, Chemicals and Fibers | 12 | |
| rubber and plastic products | 14 | |
| non-metallic mineral products | 15 | |
| Concrete, cement, lime, gypsum and others | 16 | |
| Basic metals and metal products | 17 | |
| Machinery and Equipment | 18 | |
| electrical and optical equipment | 19 |
Certification Scheme
1 Scope of application
This certification scheme is applicable to Shanghai Zhonghao Certification Co., Ltd. (hereinafter referred to as: ZOHO) to implement the medical device quality management system certification, to meet the requirements of the third-party certification system, as a standard for providing certification services. If necessary, the relevant technical requirements shall be supplemented in the certification contract.
This certification scheme shall be confirmed and adopted when the two parties sign the contract.
2 Authentication Mode
ZOHO first audit the auditee's management system for the first time, after assessment, to confirm whether the certification is approved; after certification, in the validity period of the certificate of certification of the customer's management system to monitor, confirm whether continue to meet the certification requirements.
3 Certification Process Flow Chart
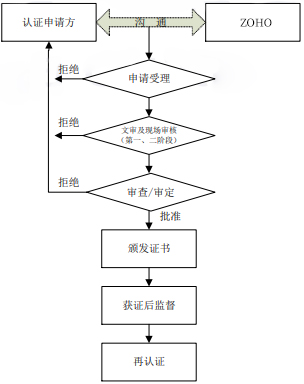
4 Application for Certification
4.1 certification applications
a. Certified customers have a clear legal status, customers have a business license, institution legal person certificate, social organization registration certificate, non-enterprise legal person registration certificate, etc., can independently apply for certification. Other types of customers should be applied by qualified units;
b. When required by the state, local or industry, the certification client shall have the prescribed administrative license documents, and the scope of application for certification shall be within the scope approved by the legal status documents and administrative license documents;
c. Certification The customer has established a documented management system according to the relevant management system standards. It has been continuously and stably operated for at least 3 months before the on-site audit of the initial certification, and has implemented at least one complete internal audit and management review or has prepared an implementation plan, and promised to continuously and effectively operate the management system within the validity period of the certificate;
d. Certification customers promise to comply with the laws, regulations and other requirements of the country, promise to always comply with the relevant provisions of certification, bear the legal responsibilities related to certification, and have the obligation to assist the supervision and inspection of certification regulatory authorities, and provide relevant materials and information truthfully for inquiries and investigations of relevant matters;
e. Certified customers are not included in the "list of serious illegal enterprises" in the national enterprise credit information publicity system ".
f. The certification organization applying for the first category of medical device production certification scope shall obtain the medical device production record certificate, and the medical device products produced shall have obtained the medical device product registration form;
g. The certification organization applying for the second type of medical device production certification scope shall obtain the medical device production enterprise license, and the medical device products produced shall have obtained the medical device product registration certificate;
h. Not accepting applications for the scope of certification for the production of Class III medical devices;
I. The certification organization engaged in the sale of Class II medical devices shall obtain the medical device business record certificate, and the medical device products sold shall have obtained the medical device product registration certificate;
j. The certification organization engaged in the sale of Class III medical devices shall obtain a medical device business license, and the medical device products sold shall have obtained a medical device product registration certificate;
k. Medical device registrants and filers do not need to provide medical device business licenses or filings when selling their registered and filed medical devices;
l. The certification customer promises to use the certification certificate, certification mark and relevant information according to the regulations after obtaining ZOHO certification, and shall not mislead the public into thinking that its products or services have passed the certification according to the contract and accept the supervision according to the regulations by using the words and symbols of the management system certification certificate without authorization;
m. Certification The customer promises to inform ZOHO of the information of management system changes and other matters that may affect the ability of the management system to continuously meet the requirements of certification standards according to ZOHO requirements after obtaining ZOHO certification, generally including: major complaints from customers and related parties; Major accidents occur; Changes in relevant conditions (including: changes in legal status, production and operation status, organizational status or ownership, compulsory certification or other qualification certificates; changes in legal representative, top management, and management representative; changes in the workplace of production, operation or service; changes in the scope of activities covered by the management system; major changes in the management system and important processes, etc.); other important situations affecting the operation of the management system occur;
n. Certification During the audit, the certification client is able to provide activities or processes related to the scope of the proposed certification.
o. Accept the witness review and confirmation review of CNAS and provide necessary support when required.
4.2 does not accept the application for certification.
a. The scope of certification applied by the certification client exceeds the scope approved by the legal status document and the administrative recognition document;
b. certification customers do not meet other relevant requirements in the 4.1 or other violations of national laws and regulations, industry regulations within the past year.
Certification Fees
Certificate Sample